living in an rna world
A single genome produces the huge diversity of cells and tissues needed to make a human by regulating gene expression to turn on and off the right genes at the right times. The final, post-transcriptional steps of gene expression — RNA processing and translation — are essential to the proper outcome. Our goal is to understand what the cell achieves by adding extra layers of regulation at these final steps, and how disruptions to this regulation can cause disease.
Our research uses bioinformatics and molecular biology to understand how post-transcriptional regulation leads to robust and flexible control of gene expression.
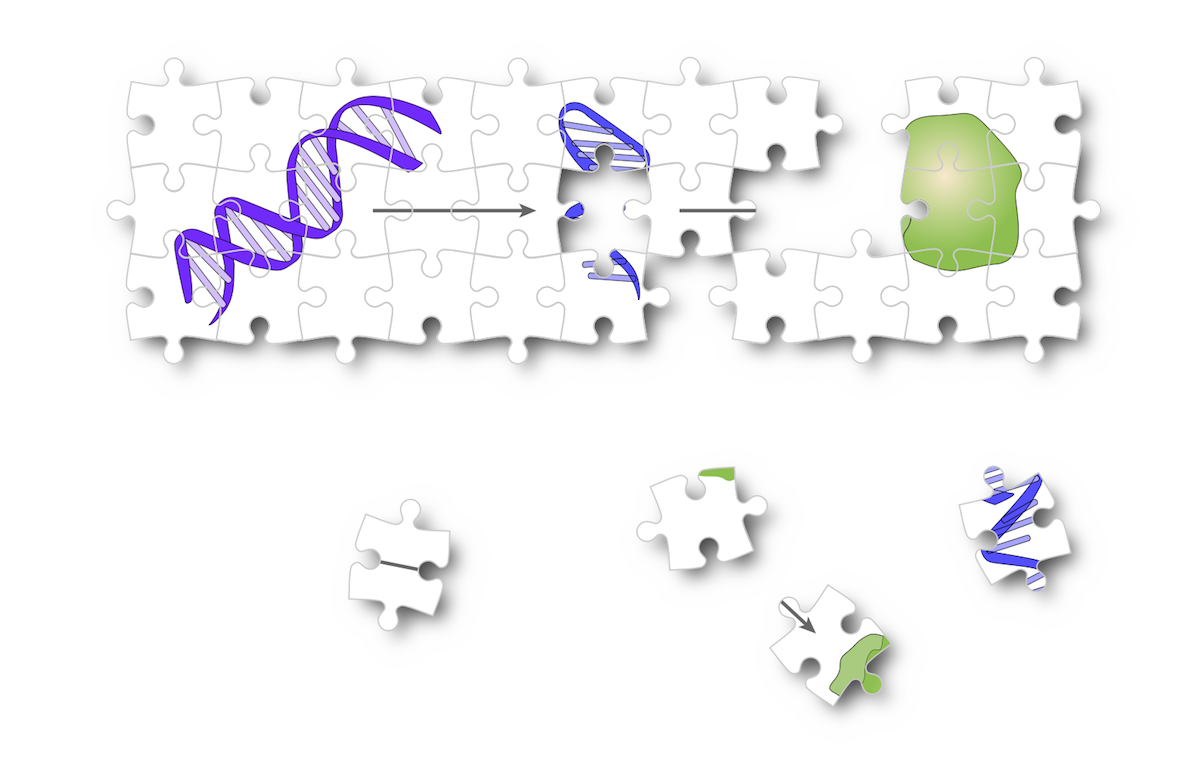
translation
One puzzle that has captivated biologists is the biased use of different synonymous codons to encode proteins. Overall, this bias likely reflects adaptation to available pools of tRNA. But, now that we can measure translation elongation in vivo, we and others have shown that the motion of the ribosome is affected by many different aspects of the mRNA sequence that it translates. We know that translation elongation does not proceed uniformly along mRNAs — what effect does that have? Tantalizing hints of consequences of different codon choice have emerged recently, such as neurodegeneration induced by mutation of a tissue-specific tRNA, or changes in expression of specific tRNAs that contribute to metastasis. Our own work has shown that simply changing the synonymous codons in a gene can dramatically change its protein expression. Our research will build on this to decode the hidden layer of regulation arising from codon choice and understand how the cell uses this to ensure the proper output of each gene.
alternative splicing
Alternative splicing shapes the transcriptome of a cell, but single cell sequencing methods have struggled to capture its impact or regulation. Previous studies led to the surprising observation that a given cell consistently produces either one or the other isoform for a particular splicing choice, with few cells producing both isoforms. We showed recently that this pattern actually arises entirely from technical limitations — alternative splicing does, indeed, lead to both isoforms in the same cell. We then showed that accounting for the true amount of information recovered can produce biologically meaningful measurements of splicing in single cells. This paves the way for reconstructions of splicing regulatory networks, harnessing the power of single cell measurements.
unproductive splicing
Another puzzle is the role of ultraconserved exon sequences in regulating gene expression. The most conserved regions of human genes are involved in a mysterious system of regulation that has been likened to connecting a printer directly to a shredder. Alternative splicing to include these ultraconserved "poison" exons can produce mRNAs that are degraded by a surveillance pathway. In my graduate work, we uncovered this system when we showed that a family of important splicing factors are auto- and cross-regulated by this process, which we call unproductive splicing. The result is a post-transcriptional decrease in expression. It is now clear that dozens of RNA-binding proteins are regulated in similar ways. The question remains: what is so important about splicing factor expression that they require such extreme sequence conservation and baroque regulation? Regulation of splicing factor levels is crucial for proper development, and over- or under-expression can lead to cancer or to diseases such as ALS. Our work will reveal how splicing factor regulation requires such extreme sequence conservation, what biological advantage arises from this system, and what consequences arise from its misregulation.